Our Solution Accelerators
Preclinical Study AI-Data Nexus
Background
The current drug discovery process involves several stages, including preclinical animal (in-vivo, ex-vivo), lab (in-vitro) studies to understand the safety and efficacy of the drug candidates. Life Sciences companies and Clinical Research organizations (CROs) generate vast amounts of data in conducting several preclinical & early discovery studies before moving the drug molecules into the clinical phase for human clinical trials. Lack of real time or interim study data limits monitoring capabilities and detecting patterns. Data is locked in discrete sources and drives, making it difficult and expensive to access and link data in meaningful ways to get a 360 view of the study.
Preclinical Data-AI Nexus
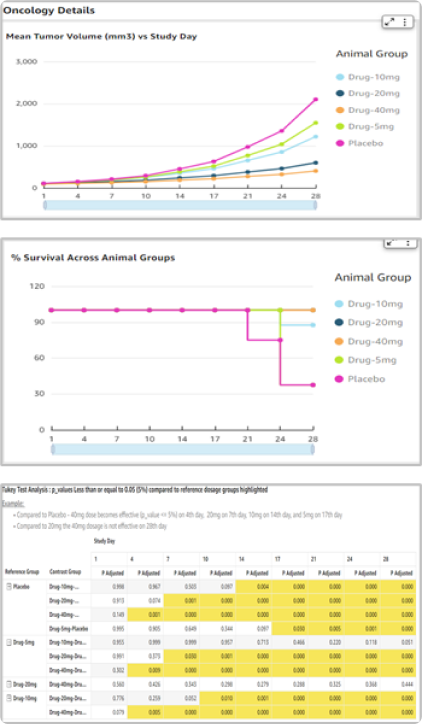

Reusable and harmonized data product from several data sources such as study plan & design, experiments schedule, dosing, ex-vivo assays etc.

Pre-built information & harmonized data models across study life cycle

Generative AI conversational capability to ask questions of studies and data

Therapeutic Area (TA) specific interactive dashboards & insights for in-vivo studies and ex-vivo assays

End-to-end automated data pipelines for integrating data into TA oriented analytical models

Data governance, and GxP validation framework and templates
Benefits
- Enhanced interim data monitoring & early signal detection
- Improved collaboration between internal and external stakeholders
- Structured repository for search across completed studies
- Improved data integrity, reduced data gaps, and efficiency gains with automated capabilities
- A projected cost synergy of 5-7% per study and 30-50% in data infrastructure
- An integrated & harmonized digital replica of ongoing & completed studies