Clinical Trial & Safety Intelligence
Background
In the face of heightened regulatory emphasis on risk-based approaches and data integrity in clinical trials,
real-time monitoring of risk and performance indicators becomes essential. Biotech, pharma, & Clinical Research organizations (CROs) rely on multiple non integrated systems and processes to record study, site, and patient data resulting in a fragmented data landscape. This necessitates concentrated efforts, effective collaboration, robust processes, and a integrated Clinical Data Lakehouse to monitor KPIs, risk indicators, and safety signals throughout the clinical trial lifecycle.
OUR SAMPLE USE CASES
Analytics Risk based monitoring, Safety Signal monitoring, Inventory & Shipment monitoring (IRT), Real world Insights
GENERATIVE AI USE CASES
HA inquiries response generator (1st draft), Secondary research conversational AI agent trained on public domain data
Clinical Trial and Safety monitoring
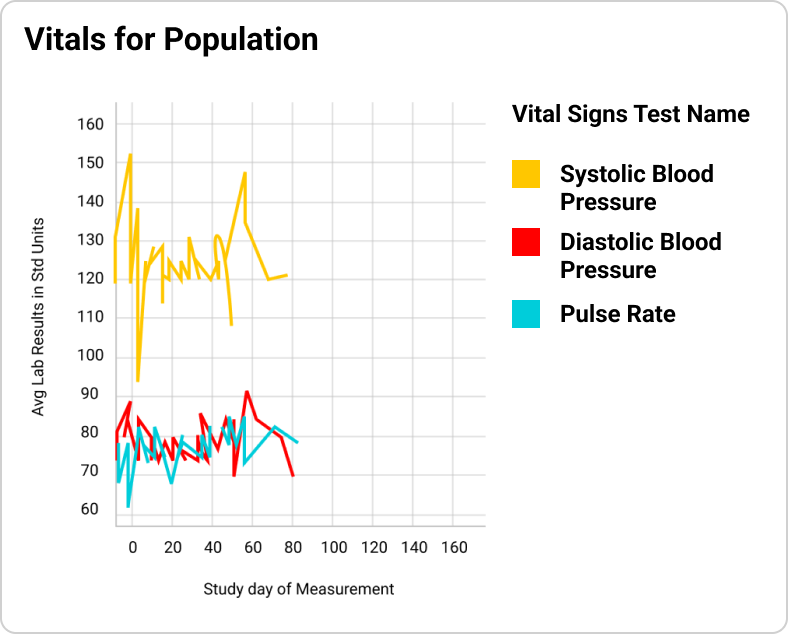

Prebuilt use cases/dashboards with several KPIs for patient, population, and labs analysis

Prebuilt library of key KPIs & KRIs for Trials Risk monitoring along with mapping of data assets

Unified information models across clinical data sources and data standards

Supply chain (IRT) data product published into harmonized analytics models for consumption
Benefits
- Enhanced interim data monitoring & early signal detection
- Improved collaboration between internal and external stakeholders
- Structured repository for search across completed studies
- Improved data integrity, reduced data gaps, and efficiency gains with automated capabilities
- A projected cost synergy of 5-7% per study and 30-50% in data infrastructure
- An integrated & harmonized digital replica of ongoing & completed studies